An educational proposal for the chemistry laboratory with historical experiences
This didactic proposal for the chemistry laboratory is intended for a basic chemistry course. The subject of the research is Prussian blue, the synthesis of Prussian blue is present on many web pages, in various versions. What they have in common is the simplicity of execution. To the synthesis of the dye is added the search for iron in the blood, an analysis linked to the constitution of Prussian blue and the synthesis of a dye other than blue obtained using copper salts. But the novelty of the didactic proposal is the reproduction of some experiences made by Macquer in 1752 which for the first time deal with the composition of Prussian blue. Here are some parts of the memoir written by Macquer, Chemical examination of Prussian blue, from 1752, relating to the description of these experiences. In this way, laboratory activity is experienced as a reconstruction of the history of chemistry which had its fundamental pivot in laboratory activity.
Laboratory experience: the analysis and synthesis of Prussian Blue dye
We reproduce some historical experiences on Prussian blue

Synthesis of Prussian blue Fe4[Fe(CN)6]3
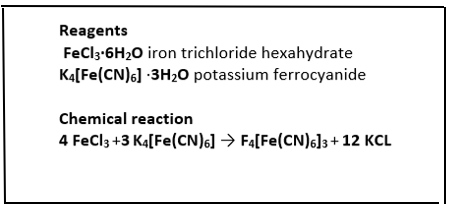
Method:
- Prepare a saturated solution of iron (III) chloride by placing 2.6 g of FeCl3 in a small beaker with 10 ml of distilled water. Use a graduated cylinder to measure the volume of water you used. Mix to dissolve.
- Separately, prepare a saturated solution of potassium ferrocyanide by pouring 2 g of K4 [Fe (CN)6] into another beaker with 10 mL of distilled water. Describe what these solutions look like.
- Prepare the Prussian Blue by pouring the potassium ferrocyanide solution into the beaker with the ferric chloride solution. Stir with a glass rod. Describe what you see happening when the solutions are mixed.
- Obtain filter paper that fits into the Buchner funnel (so that it lies flat on the bottom).
- Then pour the reaction mixture into the funnel. Scrape off all the blue product in the funnel: use a little distilled water to rinse the beaker.
- Put the filter paper on some paper towels inside the laboratory drawer and let your Prussian Blue dry. Describe what the pigment looks like, both when wet and after it has dried.
Reflections:
- Write down the reaction that is proceeding in this synthesis of Prussian blue
- Indicates many other purposes for this substance, in addition to as a dye in paints
An alternative dye with copper salts
The potassium ferrocyanide reacts with many metal salts giving characteristic colors. In place of the iron trichloride, a saturated solution of a copper salt is added to the ferrocyanide, such as copper dichloride dihydrate CuCl2 · 2H2O, obtained by dissolving 3.1 g in 10 mL in distilled water. A red precipitate is obtained.
Red blood cells contain iron
A small amount of pork or bovine liver is squeezed and washed with a few mL of distilled water. The washing water is evaporated to a small volume and then calcined in a crucible. The residue is dissolved in hydrochloric acid. A few drops of ferrocyanide solution are added to the solution. The presence of the ferric ion is revealed by the synthesis of Prussian blue.
We reproduce some historical experiences on Prussian blue
This laboratory experience on the synthesis of Prussian Blue, the first synthetic dye, is of great simplicity. It involves mixing the solutions of two reagents and observing the formation of a nice blue precipitate. In reality, originally, when the synthesis of the dye was discovered (1706), the procedure was very complex and consisted of several steps: it started with ox blood which was coagulated, then dried and mixed with potash. The mass was calcined in a stove, cooled and then taken up with water and filtered several times. The filtered solution was called the blood lye and was the key part. To obtain Prussian blue it was necessary to mix this lye with an iron salt. A series of filtering and washing followed, before reaching a marketable product. The study of the composition and structure of the dye involved several generations of chemists, starting from 1724, the year in which the procedure for its preparation was revealed. However, the first relevant work that sheds light on the composition and structure of Prussian blue is due to the chemist Pierre-Joseph Macquer (1718-1784) of an older generation than Lavoisier.

Pierre-Joseph Macquer (1718-1784)
He expounded the results of his meticulous experimental work in the magazine Histoire de l’Académie royale des sciences avec les mémoires de mathématique et de physique of 1752 in an article entitled Chemical examination of Prussian blue. Macquer’s memoir is a document of epistemological significance. It is a series of experiments that he conducts on the dye, through which, through the articulated application of a hypothetical deductive reasoning, he obtains important results. This mature and conscious use of the experimental method is all the more surprising if one thinks of the state of chemical science in his time. We are in a period that still sees Lavoisier’s revolution far from the horizon, a period in which pre-established and pre-scientific truths form the framework to which the experimental results must conform.
In his opening words, Macquer’s memory immediately makes us understand the latter’s different approach to the study of Prussian blue compared to his chemical colleagues:
All the chemists who have worked so far on Prussian blue have tried to know and perfect it only by reproducing it; and the attempts they have made have had as their object, sometimes the soapy alkalis [soda and potash] which they have tried to prepare in different ways, or by varying the quality and quantity of flammable substances [organic material such as ox blood etc.] with which the alkalis were calcined, and the degree of calcination; sometimes we tried to vary the doses of the ingredients used in the preparation of this blue, to subtract some, to add new ones, and to combine them differently: it is also the method that I followed so long, that my only purpose was to perfect Prussian blue as a dye; but when I decided to chemically examine it to find out exactly what its nature was, I followed a very different route, namely, to examine the ready-made Prussian blue, subject it to several chemical tests and try to break it down, which no one has done so far in decent way; therefore the object of this paper is, strictly speaking, the analysis of Prussian blue.
Macquer’s work is placed in a new perspective with respect to the chemistry of his time. He proceeds on the path of analysis, a non-obvious process, which demonstrates how he is starting to think in terms of simpler bodies compared to more complex bodies (A precondition for arriving at the definition of Lavoisier’s element) . It is a relevant result for someone who is placed in a pre-Lavoisier cultural framework, which still sees chemistry linked to the phlogiston theory and the theory of the four principles. The iron, for example, which enters the preparation of Prussian blue is not an element in the sense then defined by Lavoisier, iron and its oxide are not clearly distinct bodies. The experiences that Macquer conducts are numerous, some of the most significant can be chosen and re-proposed in a laboratory experience that enriches what is the simple synthesis of Prussian blue that was previously exposed. Macquer’s results were so relevant that they were inspired by Priestley, Scheele, Berthollet, Gay-Lussac and Berzelius. We follow Macquer in his attempt to analyze Prussian blue. We have produced the dye with the previous synthesis, we will use it by reproducing Macquer’s analyzes. Reading the passages in which Macquer describes and articulates his experiences has an important educational aspect. A historical context is recreated which has epistemological value, but which is also highly suggestive. We propose only some parts of Macquer’s work, the most feasible and most understandable experiences to be reproduced in a school laboratory.
He first calcined the Prussian blue obtaining a residue which has a yellow color and which is attracted by the magnet. It is ferric oxide:
I started by presenting the well-washed and dried Prussian blue with a magnet that attracted no part of it. We already knew, and M. l’Abbé Ménon mentions it in his Memoirs, that Prussian blue is not attracted to the magnet; but what we did not know, and what M. l’Abbé Ménon himself did not say, although it was something that he could think plausible, is that this same blue, being calcined, becomes quite attractive to the magnet. Here’s how I did this experiment: I put the well washed and dried Prussian blue in a crucible placed among the burning coals, vapors were formed that had a very marked odor of volatile alkali [ammonia] – a phenomenon that M. Geoffroy the young also observed in the distillation of Prussian blue – and as these vapors are exhaled, the blue color disappears to give way to a yellow color of rust or March saffron, which what remained in the crucible has always kept: this yellow powder , presented to the magnet, is attached to it entirely.
The investigation continues, subjecting Prussian blue to the action of acids:
After these first tests I made some other types, trying to break down the Prussian blue using the main chemical solvents. Although I was right to assume that the acids did not act on this blue, as it is washed with some force with acid to give it beauty and liveliness, I nevertheless believed that it would be appropriate to examine whether this type of solvent did not alter in any way, mainly when it is was helped by the heat. So I poured the three mineral acids separately on the Prussian blue, and after boiling these acids with this blue, I did not notice any appreciable alteration in this color, except that they had increased its intensity a little: these same acids , then subjected to appropriate experiments, seemed to have dissolved nothing of the Prussian blue except a little aluminous earth.
He then subjects it to the action of alkalis, such as potassium carbonate, nitre fixé par le tartre (which was obtained from raw tartar, i.e. potassium hydrogen tartrate, and dried potassium nitrate, mixed and blown up with coal to give an alkali that was basically potassium carbonate):
I believe it can be concluded from these experiments that acids have no effect on Prussian blue, even when aided by rather considerable heat. Having thus tried in vain to break down Prussian blue with acids, I resorted to alkali. I put half an ounce of this blue in a flask, and poured over ten ounces of nitro liqueur fixed by tartar [potassium carbonate]. As soon as these two substances were mixed together I saw with amazement, that without the help of heat the blue color had completely disappeared, the dust from the bottom of the flask had only a rather dull gray color: having placed this container on a sand bath to heat the liqueur until it boils slightly, this gray color has also disappeared completely; and all that was contained in the retort, both the powder and the liquor, had only a slightly reddish yellow color.
The powder is mainly composed of iron III oxide-hydroxides, of a reddish yellow color and the solution or liqueur that appears yellow is potassium ferrocyanide, according to the reaction:

In an alkaline solution, Prussian blue releases the ferric component in the form of a precipitate of hydroxide – more precisely, iron III oxide hydroxides FeO(OH) – while the yellow ferrocyanide component remains dissolved in solution. That no one had previously subjected Prussian Blue to acids and bases confirms the novelty of Macquer’s analysis-oriented approach. The effect exerted by alkalis raises new questions. It ensures that the alkali action is complete and then performs separate tests on the residual dust.
To form an opinion on the matter, I decided to examine both the yellow powder, which was only discolored Prussian blue, and the alkaline liquor which had taken away its color; and to be sure that the alkali had all the effect on Prussian blue that it was capable of producing, I poured new alkali into this already discolored and yellowed blue, I boiled it in this alkali, which was fixed nitro [potassium carbonate], the same as in the first experiment; after which I filtered the liqueur, and carefully washed the yellow powder left on the filter, until the water in which I had washed it was perfectly tasteless: I then poured strong water [nitric acid] on part of this washed powder ; there was a great effervescence, the dust dissolved, and the whole remained yellow without any trace of blue appearing. I put the other part of the yellow powder in a crucible, charred it until it turned a little red, then presented it to a magnetic iron rod which attracted it entirely, like pure iron.
The complete solubilization of the powder sample is explained by its composition of iron III oxide-hydroxides and any carbonates. A second portion of powder is instead calcined and transformed into rust-red ferric oxide.
As these experiments are complete proof that what remains of the Prussian blue after it has been discolored by the alkali is none other than iron, I concluded that the alkali decomposes this blue and dissolves everything that is not iron, and therefore I turned all my attention to this alkali which had so discolored the blue, being convinced that I would find there all that the iron lacked to be Prussian blue.
Macquer turns his attention to the solution that discolored the Prussian blue. He adds Prussian Blue until the solution is saturated. If the alkaline solution is saturated, that is, if all its alkalinity has been engaged in the reaction of Fig. 1, Macquer demonstrates this by proving the neutrality of the solution.
I filtered the liqueur, then it had a nice lemon yellow color [the color of ferrocyanide]; I tasted it, it didn’t seem to have any alkaline taste, which led me to judge that not only was the alkali loaded as much as possible with the Prussian blue coloring matter, but that this matter had caused the loss of its alkaline properties, and had, as it were, neutralized it: I mixed it, to make sure, with violet syrup [an indicator of acidity], the color of which did not change; then I poured strong water into it, and this acid did not produce the slightest effervescence, which left me no doubt that my salt, taking charge of the coloring matter of Prussian blue, had actually lost all its alkaline properties, such as I had previously suspected.
The lemon yellow solution is a ferrocyanide solution [FeII(CN)6─4]. Macquer undergoes other experiences from which he draws the indication to add an iron salt to the solution obtained previously. He uses ferrous sulphate which is the first to give a bluish precipitate of ferrous ferrocyanide which is transformed by oxidation to ferric ferrocyanide. The potassium ferrocyanide, K4[Fe(CN)6], contained in the alkaline lye, produces in solutions of ferrous salts, with complete exclusion of the air, a white precipitate of ferrous potassium ferrocyanide or ferrous ferrocyanide, depending on whether a or two molecules of ferrous salt react with one molecule of potassium ferrocyanide:
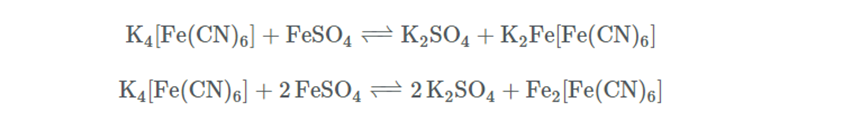
Although both of the above salts are white, a blue color is almost always obtained, because the precipitate is immediately oxidized, as seen previously in some way from the air, forming the ferric salt of ferrocyanic acid (Prussian blue):

It obtains a precipitate which is Prussian blue by adding an iron salt such as ferrous sulphate to the saturated alkaline solution of the coloring matter. He first made an analysis, now he makes a synthesis of Prussian blue .
.. so, to separate from this alkali all that had dissolved of this coloring part, it was perhaps only a matter of mixing it with a suitable quantity of sufficiently divided iron; that, finally, if this were the case, my alkali, by discolouring the Prussian blue, had become with a simple digestion an excellent lye suitable for reproducing this blue, and consequently, without any calcination, absolutely similar to what is prepared for this purpose, calcining it with flammable substances [i.e. with the traditional method]. The mixture of my alkaline liqueur with a solution of green vitriol soon cleared up my doubts; this mixture produced a mountain green precipitate, accompanied by a blue foam, just as happens when preparing Prussian blue with ordinary lye: finally a little acid poured on this precipitate, changed it entirely to an extremely bright blue .
Macquer will investigate the coloring matter contained in the alkaline solution by subjecting it to calcination and noting that:
There is also reason to believe that when this matter thus experiences the action of fire in both cases [the coloring matter, or ferrocyanide, and Prussian blue], it does not entirely disperse in vapors, but decomposes; one part is transformed into volatile alkali [ammonia] and exhales in vapors, the rest becomes entirely carbonaceous and remains strongly attached to the substance with which it was initially united: a black eye, which a sufficiently strong calcination could not remove from the alkalis, and the properties of being alterable by the magnet, acquired by the Prussian blue discolored by calcination, are proofs of what I claim.
The calcination of the residue, at red heat, which obtained after having evaporated the alkaline solution containing potassium ferrocyanide to dryness, produced volatile alkali, i.e. ammonia, and a black carbon residue that could contain graphite, iron and cementite as well as oxide of potassium. Macquer, who succeeded in isolating the ferrocyanide and calculating a residue that is attracted to the magnet as it happened for the calcination of Prussian blue, does not understand that two shares of iron are contained in Prussian blue, the first that separates by alkalinization of the same Blue as he has ascertained and the other by calcination of the coloring matter that remains in solution after the discoloration of the Prussian blue, another result he achieved. But it should also be stressed that such a deduction was not attainable even to a keen observer like Macquer.
We reproduce some experiences made by Macquer
Experience n. 1
After getting Prussian Blue from the previous synthesis, let’s take a quantity equal to a spatula tip and add it, mixing it, into a beaker containing a few mL of diluted hydrochloric acid solution. The dye remains unchanged. Diluted sulfuric and nitric acids can also be used, obtaining a similar result.
Experience n. 2
We take a spatula tip of Prussian Blue and add it, mixing it, into a beaker containing about 5 mL of saturated potassium carbonate solution. Prussian blue is discolored. Further aliquots are added until the alkaline solution is saturated. The solution should be neutral or slightly basic to the pretty indicator. The test shows, unlike experience n.1, one of the weak points of the Prussian blue dye, namely its instability in basic conditions.
Experience n. 3
From experience 2, a solution is obtained that has a yellowish color (ferrocyanide) and a yellow-orange residue (ferric hydroxide oxide). The residue is separated from the solution. Half of the residue is dissolved in an acid solution for hydrochloric acid and thr iron can be sought in the solution. The other half is calcined until rust-colored ferric oxide is obtained, which can be tested with a magnet.
Experience n. 4
Small aliquots of ferric chloride are added to one third of the yellow ferrocyanide solution. The Prussian blue precipitate is obtained.(better if in acidic conditions)
Experience n. 5
Another third of the solution is evaporated slowly until it is reduced to a small volume. It is left to dry until yellow crystals of potassium ferrocyanide are obtained.
Experience n. 6
The last third of the solution is calcined to obtain a residue that has the magnetic properties of iron.
The experiences are a reproduction of part of Macquer’s work on Prussian blue, except for n.5, which will be done by others later. Macquer ultimately separates iron III from Prussian blue i.e. ferric ferrocyanide, and then iron II from ferrocyanide. They are two different states of iron oxidation that form Prussian blue. Iron II is coordinated with the cyanide ion to form the Fe (CN)6 -4 complex. Iron III is the counter ion. Macquer, who moves within the knowledge of mid-eighteenth-century chemistry, does not possess the concept of a compound, nor is he able to distinguish a compound with two distinct forms of iron.


